Revolutionizing cartilage repair: The role of macrophages and hyaluronic acid in healing injuries
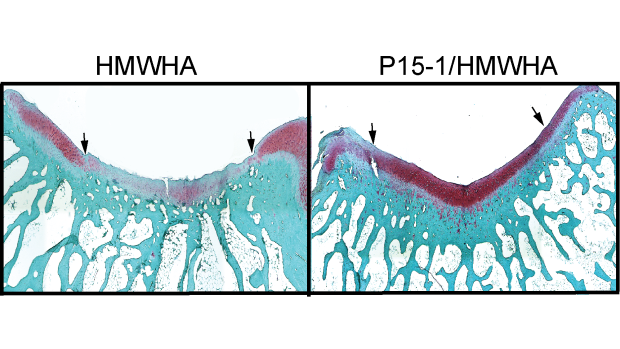
Researchers found that Intra-articular injections of P15-1 together with high-molecular-weight hyaluronan (HMWHA) improved cartilage repair. In the left image, injections with HMWHA alone produced less robust cartilage repair than the right image, where it was paired with P15-1.
Injuries of the knee resulting in damage to cartilage affect approximately 900,000 Americans annually, resulting in more than 200,000 surgical procedures. These injuries are frequently associated with pain, diminished joint functionality, and reduced life quality. In addition, these traumatic joint injuries lead to the early onset of post traumatic osteoarthritis, requiring eventual joint replacement. Joint replacement, especially at relatively young age, results in significant limitations to lifestyle and potential complications, including the limited lifespan of the implants.
Now, a new study from Professors of Biomedical Engineering and Orthopaedic Surgery Mary Cowman and Thorsten Kirsch is promising a novel solution to the issue of inflammation in such injuries, in order to promote healthy healing and prevent the need for more invasive procedures and treatments.
A key factor in the complex repair process is the body’s immune response, particularly the role of macrophages, which are immune cells that play a crucial role in inflammation following joint injury. After injury, these cells can adopt different states — pro-inflammatory and anti-inflammatory — that need to be in balance for proper repair.
Pro-inflammatory macrophages drive inflammation by releasing pro-inflammatory cytokines, which can inhibit the proliferation and viability of mesenchymal stem cells (MSCs)—cells essential for cartilage regeneration. This not only limits MSC function but also hampers their ability to modulate the immune environment and differentiate into chondrocytes, the cells responsible for cartilage formation. This can lead to chronic inflammation and the onset of post-traumatic osteoarthritis. The challenge is rebalancing these macrophages to support the anti-inflammatory varieties.
Recent research has uncovered a pivotal role for hyaluronic acid (aka hyaluronan, HA), a glycosaminoglycan found in the extracellular matrix of tissues like cartilage, in this immune modulation. While HA plays a critical role in normal cartilage tissue structure and function, and is widely used to alleviate pain in osteoarthritis, it can be degraded in inflamed tissues. Degraded HA interacts with a protein receptor known as RHAMM (Receptor for Hyaluronan-Mediated Motility), which is minimally expressed in healthy adult tissues but becomes highly upregulated following injury. While RHAMM interactions with HA are essential for processes like cell migration and tissue repair, they also contribute to inflammation and fibrosis in various pathological conditions.
Cowman and Kirsch’s new study tested the effects of disrupting these interactions, by using a synthetic peptide known as P15-1, which mimics RHAMM and competes with it for HA binding, in combination with natural high molecular weight HA. In a rabbit model for cartilage defect repair, the therapeutic formulation effectively shifts the balance of macrophages to the anti-inflammatory state, and improves healing while limiting damage.
These findings could hold promise for improving cartilage repair outcomes, offering a way to break the cycle of inflammation and promote more effective healing. As the field of regenerative medicine continues to evolve, targeting immune pathways like HA-RHAMM interactions may open new avenues for treating joint injuries and preventing the long-term consequences of post-traumatic osteoarthritis.
This work was supported by the NIH and by a grant from The Ines Mandl Research Foundation. The Ines Mandl Research Foundation (IMRF) is dedicated to providing research funding in the fight against connective tissue disease. It is the legacy of Dr. Ines Mandl (TANDON ’47, ’49), who was the first woman to graduate from the Polytechnic Institute of Brooklyn—today’s NYU Tandon School of Engineering—with a PhD in chemistry in 1949.
Bianchini, E. Sin, Y.J.A., Lee, Y.J., Lin, C., Anil, U., Hamill, C., Cowman, M.K., Kirsch, T. (2024) “The Role of Hyaluronan/RHAMM Interactions in the Modulation of Macrophage Polarization and Cartilage Repair” Am.J.Pathology 194, 1047-1061, https://doi.org/10.1016/j.ajpath.2024.01.020



